1 Which of the Following Is an Arrhenius Acid
Hydrochloric Acid HCl. Indicate whether each of the following statements is characteristics of an Arrhenius acid Arrhenius.

Solved 1 Which Of The Following Is An Arrhenius Acid Chegg Com
According to Arrhenius an acid is a substance that dissolves in water to produce H ions and a base is a substance that dissolves in water to produce hydroxide OH ions.
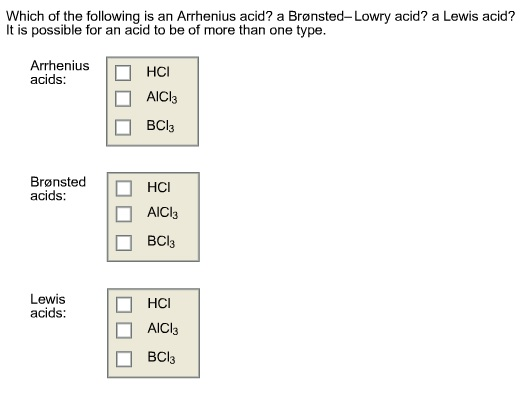
. 2 Which of the following is an Arrhenius base. NH3 HI 2 NH I b. 2 Arrhenius acids are ionic compounds and Brønsted-Lowry acids are covalent compounds.
All of these are Arrhenius bases. C H 3 P O 4 H 3 P O 4 H 3 P O 4 H H 3 P O 4 is an Arrahenius acid because it produced H ion. Which of the following is the strongest base.
Some other examples of Arrhenius acids are Hydrofluoric acid HF Nitric acid HNO 3 Hydrobromic acid HBr Sulphuric acid H 2 SO 4 Sulphurous acid H 2 SO 3 Perchloric acid HClO 4 Phosphoric acid H 3 PO 4 Hydroiodic acid HI Carbonic acid H 2 CO 3 etc. NH3 2SO2 3AlCl3 4HNO3 1 See answer r303045 is waiting for your help. 1 The result of salt hydrolysis in aqueous solution is always a basic solution.
Which of the following are Arrhenius. A CaCO3 H2O CaOH2 CO2 b C3H8 5O2 3CO2 4H2O c 2MgNH4PO4 Mg2P2O7 2NH 3 H2O. HCl aq Cl - aq H aq HNO 3 aq NO 3- aq H aq The formation of the H cation in water is responsible for all the properties of an acid.
Which of the following is a monoprotic Arrhenius acid. Following a Connecting Activity the teacher performs a demonstration on two unknown clear liquids to determine the pH before and after mixing. AlOH3 3HNO3 Al3 3H2O 3 NO3III.
Which of the following is an Arrhenius acid. A CH3CO2H B NaOH C CH3OH D LICI E More than one of these compounds is an Arrhenius base. Produces H ions in water d.
Which acts as a Lewis acid but neither Arrhenius nor Brønsted-Lowry. Na2O I think c or d. NHO 3 aq H 2 O l H 3 O aq No 3.
It is n Arrahenius bases since it produced O H i o n. Identify the Arrhenius acids among the following. Is an electrolyte 2.
D H I H I H I t H I is an Arrhenius acid because it produced H ion. 3 Each successive step in the dissociation of a polyprotic acid. Identify the Bronsted-Lowry Acids and bases in these reaction and group them into conjugate acid-base pairs.
A reaction may fit all two one or none of the categoriesI. Which of the following is an Arrhenius base. Has a sour taste b.
Which of the following is an Arrhenius acid. In this reaction nitric acid dissolves in aqueous water to give hydrogen and nitrate ions. Typical Arrhenius acids are hydrochloric acid and nitric acid since they undergo the following reactions in water.
Cu2 4 Cl CuCl42II. HCl aq H 2 O l H 3 O aq Cl aq Other examples of Arrhenius acids are listed below. The trick to recognizing an Arrhenius acid is to look for a molecule that starts with an H and typically contains an oxygen or halogen.
In the pure state Arrhenius acids are covalent compounds. Simply put a proton donor. A compound that increases the concentration of hydroxide ion OH in aqueous solution.
In the pure state Arrhenius bases are ionic compounds. For each of the following chemical reactions calculate the maximum mass of the underlined product that could be produced from 1000 g of the underlined reactant. Pearson Chemistry Matta Staley Waterman Wilbraham.
In the reaction below AH is an avid BOH is a base reacting together to form a saltA-B and water only. Kaypeeoh72z and 42 more users found this answer helpful. Dissociation is the process by which Arrhenius acids produce H ions in solution.
Acidic strength increases with. Acids Bases Unit Lesson 1. Physical Science Concepts In Action 2nd Edition Frank Wysession Yancopoulos.
Select the correct statements. H 2 S O 4 acts as a base in presence of H C l O 4. Which of the following is an Bronsted-Lowry acid.
Identify the following as an Arrhenius acid or a base a. Correct answer to the question Classify the following as Arrhenius Bronsted-Lowry or Lewis acid-base reactions. Perchloric acid is stronger acid than H 2 S O 4.
Common examples of Arrhenius acids include. A H2SO4 B LIOH C NH2CH3 D CH3CH3 E More than one of these is an Arrhenius acid. An Arrhenius acid is a molecule that when dissolved in water will donate an H in solution.
C a O H 2 is not an Arrhenius acid. Hydronium ion a OH- b H30 c donates an H d accepts an H e produces OH- in water f produces H in water. Match of the following 1Arrhenius base 2Arrhenius acid 3Brönsted base 4.
Greater the value of p K a more is the acidic strength. In other choices CaO or calcium oxide and K2O or potassium oxide are examples of ionic compound while NH3 or ammonia is an example of covalent compound. All the following act as a Lewis acid in water except a.
We get result where a H 2 O c H 3 P O 4 4 H I are Arrhenius acids. The reaction of an acid and a base. Keeping it similar to the general acid properties Arrhenius acid also neutralizes bases and turns litmus paper into.
Which of the following statements concerning Arrhenius acids and Arrhenius bases is incorrect. In the following choices of compounds HCl or hydrogen chloride or hydrochloric acid is an example of Arrhenius acid. Arrhenius Theory Students Lesson at a Glance Lesson Summary This lesson contains four activities that overview the Arrhenius theory of acids and bases.
All of these are Arrhenius acids. An unknown solution forms a precipitate when the pH is raised but does not when sulfate is added. A compound that increases the concentration of hydrogen ion H in aqueous solution.
Indicate whether each of the following statements is characteristic of an Arrhenius acid Arrhenius base or both. Is named barium hydroxide e. Arrhenius acid act as a good electrolyte as it dissociates to its respective ions in the aqueous solutions.

Solved Which Of The Following Is An Arrhenius Acid All Of Chegg Com

Solved Which Of The Following Is An Arrhenius Acid A Chegg Com

Solved Which Of The Following Is An Arrhenius Acid H2so4 Chegg Com
Comments
Post a Comment